Background science for the Turkish mutations, II.
In the last post we discussed the dense canopy of sugars linked to cell surface proteins that covers most cells. This outer fur-like sugar surface is called the glycocalyx and plays an important biological role, including cell-cell recognition and communication, interacting with and binding of cells to the material that glues cells together (the extracellular matrix), altering or modulating the response of immune cells and proteins, and, most important for our purpose, protecting against or determining sensitivity to pathogens like the flu virus. There is a lot of technical detail here that is probably more than most readers want to know. But there is a solid subset of readers of this blog who have a genuine appetite for being able to read the flu literature, and this is for them. It won't make you an expert, but it should help understand the language.
Here's the picture again (go back to the first post for a fuller explanation):

The proteins with the sugars attached that make up the glycocalyx are the black threads (the proteins) with the colored hexagons attached to them (the sugars). There are quite a few different sugars so there can be many different patterns. We are primarily concerned with what is going on at the very tips of the sugar chains, where we commonly have a special sugar called sialic acid. Sugars are molecules made up of carbon, hydrogen and oxygen, where each carbon has either an oxygen attached to it with a double bond or a hydroxyl group (-OH) and a hydrogen. Table sugar (sucrose) has 12 carbons (made up of two simpler 6 carbon sugars hooked together), and so on for a vast array of possible sugars. Sialic acid has 9 carbons.
So far we haven't done much except point out that we are particularly interested in a particular sugar, sialic acid, that often rests at the tips of the sugar chains that come off the glycoproteins or glycolipids that are part of the cell membrane (the cell surface). Unfortunately at this point we need to get into some of the details of the chemical structure of sialic acid in order to explain what terms like α-2, 3 or α-2, 3 linkages mean. The linkages are important determinants for whether a bird virus will think it is seeing a human cell or a bird cell.
So here are some of the grubby details. You will see below that the particular form of sialic acid we are most concerned with has a slight modification, an acetyl group attached through a nitrogen at carbon 5 and thus it is also called Neu5Ac, because confusingly, this sialic acid is also called neuraminic acid. So Neu5Ac and "sialic acid" are the same and you will see both names in the literature, often in the very same paper. This terminology is a bit clearer if you see the chemical structure of sialic (N-acetylneuraminic) acid, so here it is (you don't need to know it to follow what comes later, however. This is for people who want to know what the trms mean and why they are of interest. The pics are from the website of Dr. Kevin J. Yarema at Johns Hopkins). [NB:It is common in drawing these structures to leave out the letter "C" for carbon which is assumed to be wherever two lines meet (so carbon #2 is the spot just to the right of the -O-, or oxygen atom, in the chair-shaped ring in the middle of the picture]:


This looks more complicated that it is. The (black and red) sugar carbon chain has bent back on itself and formed a ring that has an oxygen in it. Because of the way atoms connect to each other, the ring is also slightly deformed into a shape like a chair. The green modification addition is the N-acetyl group attached at the carbon numbered #5, hence Neur5Ac. The part on the right hand side that is labeled Carbon #2 (the anomeric position) is where the sialic acid attaches to the rest of the oligosaccharide (multi-sugar) chain. It turns out there are two ways to make this attachment, depending on whether the attachment is above or below the plane of the chair-shaped ring structure. These two ways to link are called α- and β- linkages. The α-linkage connects below the plane and that's the one that humans have on their glycocalyces.
For those who are following the bird flu story, you already know that the influenza virus likes α-2, 6 sialic acid linkages while the bird virus likes α-2, 3 sialic acid linkages. So we are two thirds of the way to explaining this since we know that the "2" refers to attachment at the carbon #2 in the ring while the α part refers to whether the linkage is above or below the plane of the ring. The other number in α-2, 6 or α-2, 3 (the 6 and the 3) is the number of the carbon atom on the sugar that the sialic acid links to via the α-linkage. The sugar important for us is called galactose, which has 6 carbons. The sialic acid at the tip of the chain can be linked to the next in line almost-at-the-tip galactose either at galactose's carbon #6 or its carbon #3.
Here is what the sialic acid - galactose linkage looks like, on the left a 2, 6 linkage and on the right both a 2, 8 [we didn't discuss this] and a 2, 3 linkage.

The letter R on the right of the galactose unit stands for the rest of the oligosaccharide, a chain of variable amounts of sugar units that ends at the protein's amino acid. If you compare the shapes of the α-2, 6 and the α-2, 3 linkages (ignore the α-2, 8 part) you will see that they are different, one is straighter and one is slightly bent, and it is those differences that the HA protein of the bird influenza versus the human influenza virus notices when it is looking for a place to grab on to.
The whole assembly: [sialic acid] connected to [galactose] connected to [remaining oligosaccharide] connected to the membrane anchored [protein] is called a glycoprotein. Here is a cartoon picture of sialic acid tipped glycoproteins (it's hard to see but those are all little "Sia" on the leaves of the tree in the middle):
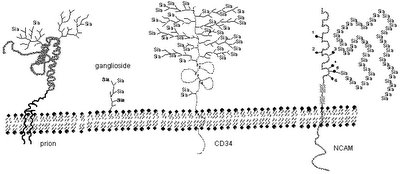
The sialic acid is the thing the influenza virus is looking to grab onto via its hemagglutinin (HA) protein spike that protrudes from its surface (for additional explanation of the viral proteins, see our write-up, Influenza Primer II at The Flu Wiki).
As an analogy, the virus is looking for a specific "street address" of the house that has the kind of lock it has a key for. It knows the number is "34" (sialic acid) and that it is on a green background (attached to a galactose) and that it is on the right side of the door (as opposed to being above the door or to the left of the door or somewhere else). The orientation with respect to the door is like the linkage (α 2, 3 or 2, 6 or 2, 8 for example). When it sees the right address it rings the bell (and other things happen which we haven't discussed but which lead to the door opening).
By the way, the alternative name for sialic acid, neuraminic acid, might remind you of the name of the other protein spike on the flu virus, neuraminidase. The neuriminidase protein on the virus is an enzyme, and any enzyme with the suffix -ase indicates it splits things off, in this case neuraminic or sialic acid (it is also sometimes called a sialidase). We'll see more of this in a later post (I hope).
Here's a summary. What we have done so far is explain (in the first post) what the surface of a cell looks like to a virus (it is covered with a pattern of sugar chains that the cell uses for a variety of purposes) and then in this post looked in more detail at a common sugar at the tips of some of these sugar chains that the flu virus is "looking for" as a place to grab onto preparatory to entering a cell and infecting it. That sugar, sialic acid, has a numbering scheme and a shape and attaches to another sugar (galactose) closer in through its own #2 carbon to either the #3 carbon (birds) or #6 carbon (humans) via a link that hooks in below the plane of the sialic acid ring (an α-linkage). The difference between hooking to either #3 or #6 of galactose makes the sugar chain look different to the virus.
There is still more to this story, however, which will have to wait for another post.
For those who are following the bird flu story, you already know that the influenza virus likes α-2, 6 sialic acid linkages while the bird virus likes α-2, 3 sialic acid linkages. So we are two thirds of the way to explaining this since we know that the "2" refers to attachment at the carbon #2 in the ring while the α part refers to whether the linkage is above or below the plane of the ring. The other number in α-2, 6 or α-2, 3 (the 6 and the 3) is the number of the carbon atom on the sugar that the sialic acid links to via the α-linkage. The sugar important for us is called galactose, which has 6 carbons. The sialic acid at the tip of the chain can be linked to the next in line almost-at-the-tip galactose either at galactose's carbon #6 or its carbon #3.
Here is what the sialic acid - galactose linkage looks like, on the left a 2, 6 linkage and on the right both a 2, 8 [we didn't discuss this] and a 2, 3 linkage.

The letter R on the right of the galactose unit stands for the rest of the oligosaccharide, a chain of variable amounts of sugar units that ends at the protein's amino acid. If you compare the shapes of the α-2, 6 and the α-2, 3 linkages (ignore the α-2, 8 part) you will see that they are different, one is straighter and one is slightly bent, and it is those differences that the HA protein of the bird influenza versus the human influenza virus notices when it is looking for a place to grab on to.
The whole assembly: [sialic acid] connected to [galactose] connected to [remaining oligosaccharide] connected to the membrane anchored [protein] is called a glycoprotein. Here is a cartoon picture of sialic acid tipped glycoproteins (it's hard to see but those are all little "Sia" on the leaves of the tree in the middle):

The sialic acid is the thing the influenza virus is looking to grab onto via its hemagglutinin (HA) protein spike that protrudes from its surface (for additional explanation of the viral proteins, see our write-up, Influenza Primer II at The Flu Wiki).
As an analogy, the virus is looking for a specific "street address" of the house that has the kind of lock it has a key for. It knows the number is "34" (sialic acid) and that it is on a green background (attached to a galactose) and that it is on the right side of the door (as opposed to being above the door or to the left of the door or somewhere else). The orientation with respect to the door is like the linkage (α 2, 3 or 2, 6 or 2, 8 for example). When it sees the right address it rings the bell (and other things happen which we haven't discussed but which lead to the door opening).
By the way, the alternative name for sialic acid, neuraminic acid, might remind you of the name of the other protein spike on the flu virus, neuraminidase. The neuriminidase protein on the virus is an enzyme, and any enzyme with the suffix -ase indicates it splits things off, in this case neuraminic or sialic acid (it is also sometimes called a sialidase). We'll see more of this in a later post (I hope).
Here's a summary. What we have done so far is explain (in the first post) what the surface of a cell looks like to a virus (it is covered with a pattern of sugar chains that the cell uses for a variety of purposes) and then in this post looked in more detail at a common sugar at the tips of some of these sugar chains that the flu virus is "looking for" as a place to grab onto preparatory to entering a cell and infecting it. That sugar, sialic acid, has a numbering scheme and a shape and attaches to another sugar (galactose) closer in through its own #2 carbon to either the #3 carbon (birds) or #6 carbon (humans) via a link that hooks in below the plane of the sialic acid ring (an α-linkage). The difference between hooking to either #3 or #6 of galactose makes the sugar chain look different to the virus.
There is still more to this story, however, which will have to wait for another post.

<< Home